How to capture ICSRs for COVID-19 treatments
In order to better understand the efficacy and safety of treatments used against COVID-19, it’s crucial that countries share relevant adverse event reports in a timely manner. UMC provides this coding guidance for reports concerning COVID-19 treatments.
It is a global concern that safety information regarding the different treatment alternatives for SARS-CoV-2 infection is limited. It is important to share information on suspected side effects from any of the medicines used to treat COVID-19, as well as how medicines taken by patients to manage long-term, pre-existing conditions are affected by the infection and treatments used.
Given the global scale of the pandemic, we should make every effort to minimise delays in reporting related to COVID-19, so that countries can benefit from each other’s experience as soon as possible.
UMC encourages the use of common coding principles to support global analysis of incoming data, and has issued the following guidance. UMC has implemented the updated MedDRA 23.0.
Coding guidance
MedDRA has updated its 23.0 release, see recent news from MSSO and their Excel spreadsheet with COVID-19 related terms. To support the retrieval and analysis of ADR reports specifically related to COVID-19 treatments, our most important advice is to code the indication of use for the suspected drug using the appropriate COVID-19 related terms available in MedDRA.
In addition to demographic details, such as the sex and age of a patient, other details that are particularly useful for analysing and identifying COVID-19 related cases for analysis are:
- relevant medical history (including concurrent conditions)
- outcome of the reaction
- results of tests and procedures
- cause of death (if applicable)
- narrative, sender’s diagnosis, and sender’s comments
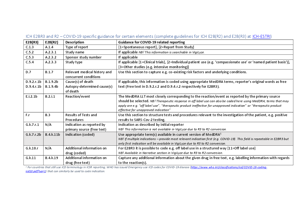
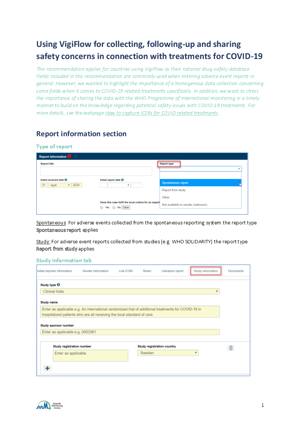
When treatment is given as part of an approved clinical trial for COVID-19, appropriate fields should be used to indicate this noting the study name and study number. The report type should also indicate Report from study.
When treatment is given for unapproved indications, or off-label use, the therapeutic response can also be captured using MedDRA; terms that may apply include “off label use”, “therapeutic product ineffective for unapproved indication” and “therapeutic product effective for unapproved indication”. These terms can be used together with applicable adverse reaction terms to describe the individual case. Off-label use can also be captured as additional information on the drug.
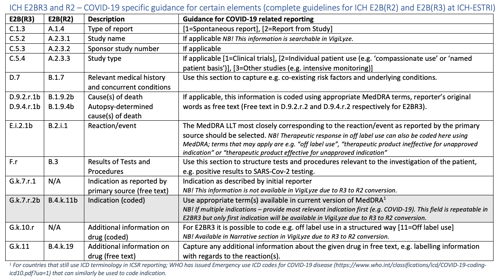
View the table “ICH E2BR3 and R2 – COVID-19 specific guidance for certain elements” in full resolution below.
Guidance on using VigiFlow
For guidance on how to best utilise VigiFlow in the COVID-19 context, read the document “Using VigiFlow for collecting, following-up and sharing safety concerns in connection with treatments for COVID-19” below.