Your one-stop shop for vaccine safety surveillance
VigiFlow for AEFI is purpose-built for collecting and analysing reports of possible side effects from vaccines.

Want to
know more?
Write to us at
support@who-umc.org
Monitoring adverse events following immunisation (AEFI) is essential to vaccine safety. VigiFlow for AEFI has been specifically developed for national immunisation programmes to manage and analyse vaccination data.
UMC expanded its pharmacovigilance data management system to capture AEFI data during the pandemic. Up until that point, VigiFlow had mainly been used by countries in the WHO Programme for International Drug Monitoring to collect and analyse reports of potential side effects to medicines. Working closely with WHO, UMC also developed a standalone system for immunisation programmes to meet the new regulatory guidelines on vaccine safety surveillance.
In VigiFlow for AEFI they now have a one-stop shop for all their vaccine safety surveillance needs, from event reporting, investigation and analysis to data sharing.
Want to boost your AEFI reporting?
Key benefits
- Based on the standard reporting form for AEFI, VigiFlow for AEFI includes the 25 core variables recommended by WHO for collecting AEFI data.
- Structured data entry with built-in WHO GIS data mapping improves data collection, reducing the need for data cleaning while speeding up the data analysis process.
- Evaluate vaccine adverse event reports at national and subnational levels.
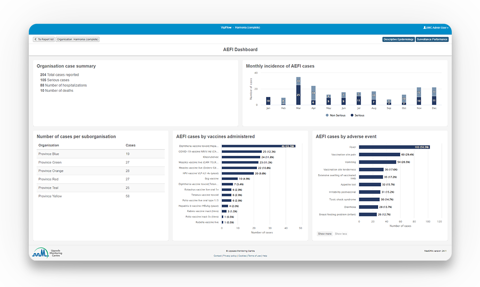
- Structured geographical data displayed visually in graphs and dashboards helps you quickly gain insights for decision-making.
- One-click sharing to WHO’s global database of adverse event reports – VigiBase.
VigiFlow for AEFI and VigiMobile
Immunisation field workers can quickly and accurately report AEFI on their smartphones or other hand-held devices even when they are offline using VigiMobile. VigiMobile contains known adverse events and approved vaccine lists. It also uses WHO GIS technology to provide structured geographical data such as local vaccination administration and patient data at time of reporting to help investigators detect and respond quickly to vaccine-associated events. All reports are sent directly to VigiFlow for AEFI where they can easily be analysed by the programme manager.
How does VigiFlow for AEFI differ
from VigiFlow?
While VigiFlow can be used for reporting side effects from medicines as well as vaccines, VigiFlow for AEFI has been optimised for organisations working solely with vaccine safety surveillance.
Using WHO's standard reporting form for AEFI as an interface, VigiFlow for AEFI streamlines workflows for processing and analysing AEFI data.
How much does it cost?
VigiFlow for AEFI is free of charge for eligible organisations.
For access and more information, contact: support@who-umc.org.
The COVID-19 pandemic led to an increased focus on strengthening vaccine surveillance systems globally. UMC, under the guidance of WHO, has worked with WHO PIDM member countries to support them with training, tools and guidance on how to improve their systems, by collecting and analysing AEFI (adverse events following immunisation) reports. This film looks at why vaccine surveillance is so important, the challenges posed by COVID-19 and what UMC has done within the WHO PIDM to develop and improve vaccine safety.
Video
In the spotlight: Vaccine safety in the WHO PIDM