Your direct channel for post-licensing safety reporting
VigiFlow eReporting for Industry makes it faster and easier for regulatory authorities to collect fully ICH-compliant post-marketing and clinical trial safety reports from marketing authorisation holders.

Want to know more?
Write to us at
support@who-umc.org
One important source of information on suspected side effects from medicines is the safety reports that come in from manufacturers once their product is on the market. Getting that data quickly is key to making sure safety issues are detected early.
In many countries paper or email reporting is still the rule due to a lack of common infrastructure for exchanging data electronically. This is a challenge, especially for countries looking to strengthen their regulatory systems and meet the technical requirements for membership of the International Council for Harmonisation (ICH). Under ICH guidelines it is mandatory for countries to have systems in place that are compatible with ICH E2B(R3) – the international format for exchanging information on the suspected side effects of medicines.
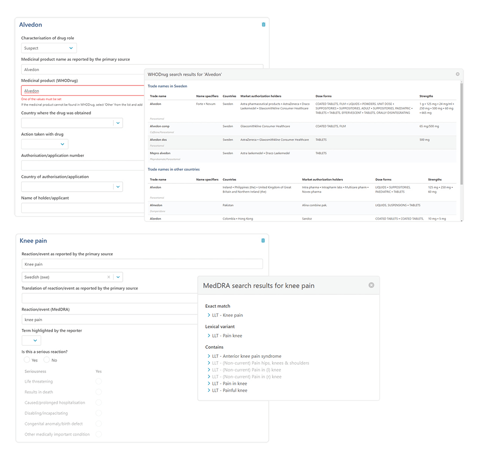
As more and more countries apply for ICH membership, VigiFlow is evolving in step with quality guidelines. VigiFlow eReporting for Industry is an add-on module to VigiFlow that makes it easier for regulatory authorities to get the data they need, how they want it. It increases the quantity and quality of information you receive by helping companies to report in a standardised way, saving you time on manual data entry and coding to WHODrug Global and MedDRA. Removing your coding burden frees up more resources for signal detection and speeds up decision-making.
VigiMobile
Based on WHO’s standard reporting forms for medicines and vaccines, VigiMobile forms capture all the core variables recommended by WHO for evaluating adverse event data.
Key features
- Off-the-shelf solution – low implementation costs and no installation necessary.
- With VigiFlow eReporting for Industry all marketing authorisation holders can achieve compliance with ICH guidelines, regardless of whether their databases are ICH compatible.
- Marketing authorisation holders report directly to you using manual data entry or ICH-compliant E2B xml files.
- Built-in WHODrug and MedDRA coding support eliminates the need for manual coding and data entry, freeing up resources for analysis.
- Moving coding to marketing authorisation holders increases the accuracy of the information you receive, reducing the risk of errors and removing the need for cross-checking.
Got a licence?
VigiFlow eReporting for Industry comes at an additional cost to VigiFlow. Contact us to find out how you can get started at support@who-umc.org
Before VigiFlow and Industry eReporting, we had to enter safety reports manually from emails received from industry into our own system which crashed often, and was not ICH E2B compliant.
Maria Victoria Urrea Duque Pharmacovigilance officer at the Colombian National Institute for Medicines and Food Surveillance (INVIMA)
Marketing authorisation holders, meanwhile, benefit from streamlined processes and improved workflows. Minimal implementation is required for them to integrate VigiFlow eReporting for Industry with their own systems so that safety reports comply with the ICH guidelines.
This not only helps them to follow the specific regulatory requirements for reporting in their own country but also simplifies data sharing across borders as more countries make the ICH guidelines mandatory.
VigiFlow eForms
Our web forms make it easier for patients and health workers to give national centres the information they need on suspected side effects to medicines and vaccines.