WHODrug Koda – the coding engine
WHODrug Koda is an automated coding service custom-built by UMC. The service is specifically designed for increasing the efficiency, consistency and quality of drug coding, with the end goal of safer use of medicines.

UMC recognises that the vital activity of drug coding can be both challenging and time consuming. The ever-expanding number of drugs available on the global market will increase the need for automated services for drug coding.
WHODrug Koda is an automated coding engine developed in response to this need: it combines the use of artificial intelligence by machine learning, thorough and continual training of the engine, industry best practices, and UMC’s accumulated insights on drug coding.
Key features
- It enables automated coding of drug names (including non-unique names).
- It provides automated ATC selections according to the latest regulatory expectations.
- It is possible to integrate WHODrug Koda in existing coding tools or similar via an API.
- Also available as a web application, it is easily accessible via the UMC website.
Coding with WHODrug Koda
Watch the video below to find out how WHODrug Koda can save you a mountain of work, deliver near perfect results time after time, and slot seamlessly into your coding operation.
✓ Efficiency
✓ Quality and consistency
✓ Usability
How to access WHODrug Koda
The service is available as in the standard WHODrug Global subscription and is provided as: (1) an API, which can be integrated in your existing coding tool or similar, or (2) an intuitive web application.
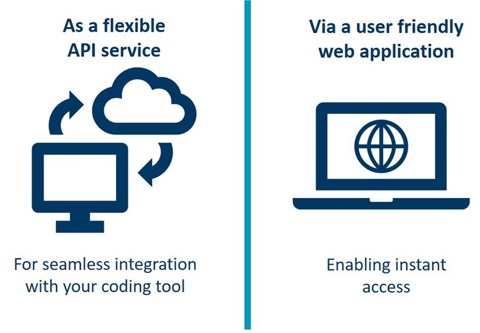
1) Access the Koda API. Software vendors approved by the WHODrug vendor programme can implement the Koda API into their respective platforms. Consult with your software provider to have Koda incorporated in your platform.
2) Access the WHODrug Koda web app. All users of WHODrug can access the WHODrug Koda web application.
If you have any questions, do not hesitate to contact us.
